Bluenote Launches the First Agentic Regulatory Platform for Drug & Device SubmissionsRead more
Bluenote
Bluenote
Bring your life-saving
therapies to patients sooner
Accelerate regulatory submissions by 50-75% with advanced AI for life sciences. Trusted by the industry’s largest pharma, medical devices, diagnostics, CROs and CDMOs.
Developed by experts in AI and life sciences, built for leading companies
Focus your resources on science—not busywork—with a platform that automates your most critical workflows.
Produce first drafts of scientific and regulatory documents instantly — aligned to your templates, SOPs and global guidelines, with built-in verification and traceability.
Free teams to focus on strategic work. Refine data presentations, format datasets and tables, create figure captions, and run gap analyses.
Workflow Builder
From hours to minutes: agents automate repetitive, multi-step workflows so scientists and SMEs can focus on innovation.
Search across internal data in seconds. Surface insights fast, reduce duplication, and unlock decades of institutional knowledge across teams.
Translate your technical, regulatory, and marketing documents while using your glossary and preserving your formatting and styling.
Grounded in your data and standards
Every document, for every stage of development
Extend automation across Pre-Clinical, Clinical, CMC, Regulatory, Quality, and Commercial—accelerating documentation across the entire product lifecycle.
- IND, NDA, BLA modules
- Clinical Study Reports (CSRs)
- Nonclinical Study Reports
- Nonclinical Written and Tabulated Summaries
- Investigator Brochures (IBs)
- Responses to Health Authority Queries (RTQs)
- Validation Reports
- CMC Documentation
- Safety Documentation
- Protocols, SOPs, and Briefing Books
- Informed Consent Forms
- CAPAs and Deviation Reports
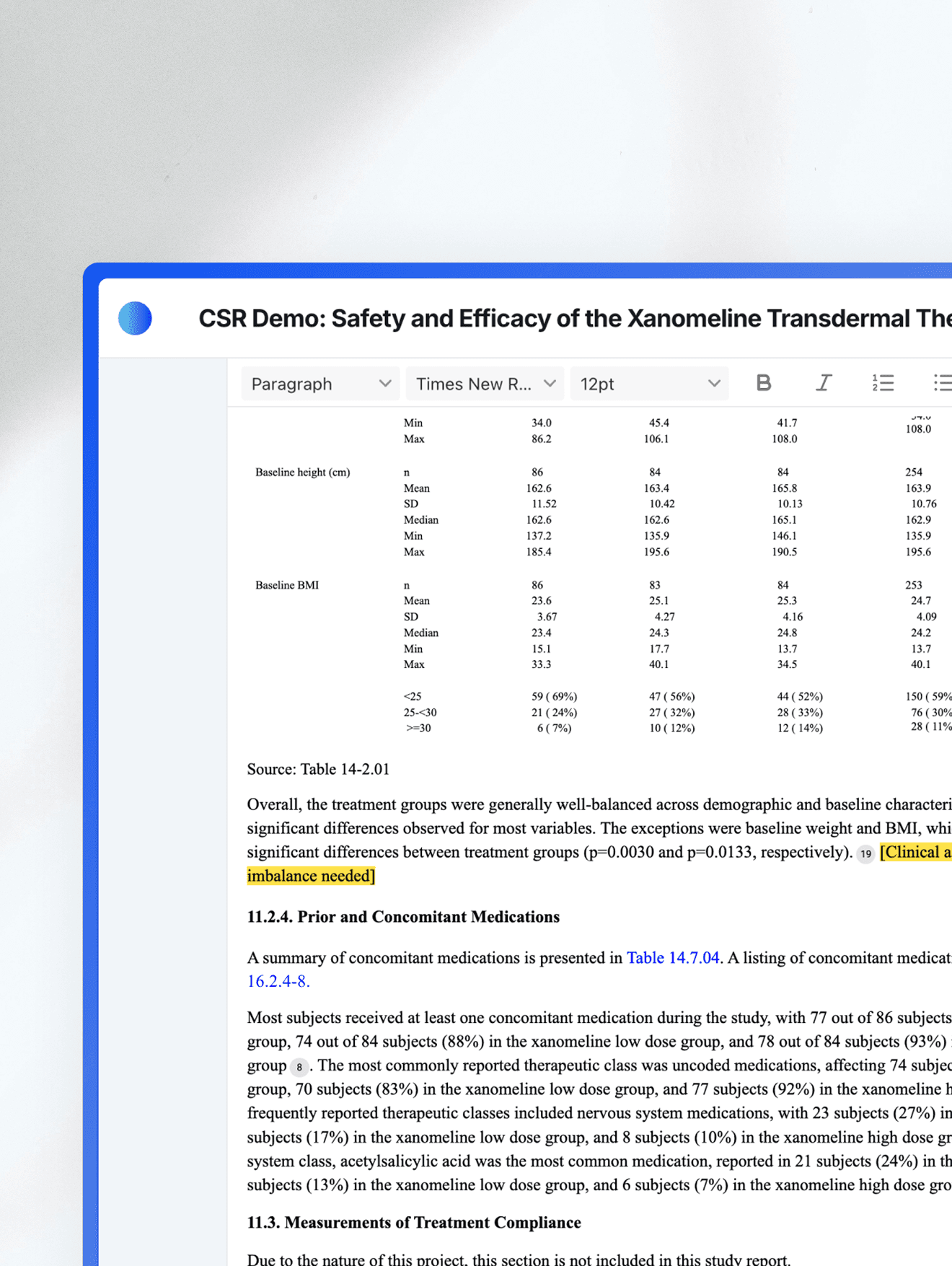
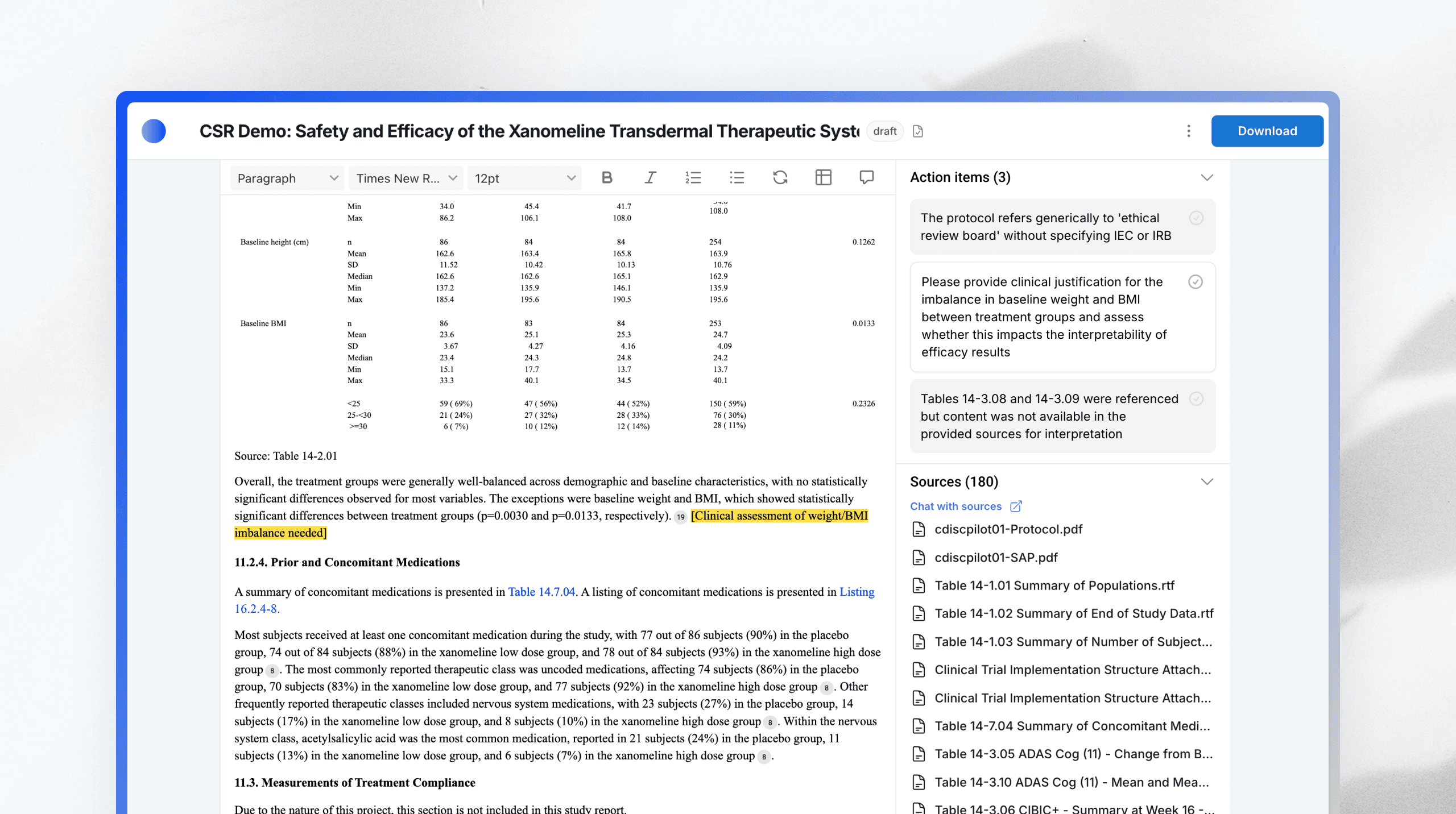
Where AI transformation meets enterprise-grade control
Security and Compliance
Bluenote adheres to leading standards, such as SOC 2 Type II and HIPAA, and offers enterprise-grade features including SAML SSO, IP allowlisting, and more.
No training on Customer Data
Your data is not used to train models. Zero data retention policy with LLMs. You retain complete control over your data.
Support for on-premise deployment
Host the platform and AI models within your own IT infrastructure for maximum security and control.